|
Plastic is made by combining monomers into polymers under
great heat and pressure in a process called polymerization. Each manufacturer
has its own proprietary formula for each plastic. And each uses a variety of
additives such as plasticizers for flexibility, UV filters for protection from
sunlight, antistatic agents, flame-retardants, colorants, antioxidants, and
more. Heavy metals such as cadmium, mercury, and lead are common additives.
There are also chemicals used to facilitate production such as mold releases,
and countless other toxic chemicals regularly added to plastic consumer goods
without our knowledge or approval. Many of the products and byproducts of the
intermediary steps of plastics production are used in other plastics or
industrial processes and products such as pesticides or fertilizer. For holistic
thinkers, the mention of plastics and pesticides in the same sentence should
begin an informative thought process, while keeping in mind that they all have
complete regulatory approval.
Antioxidants
Antioxidants are development,
manufacture and sold for use in feed, food and industrial users.
Oxidation is the major cause of
deterioration of food and feed products which contain fats and oils, because it
is one of the main causes of rancidity in these products. Oxidation is a "weak
point" of almost all fats and vegetable oils, even those containing natural
antioxidants.
The factors to consider, when
discussing deterioration of fats and oils and methods of prevention, include
biochemical processes (enzyme caused deterioration), chemical processes (oxygen
caused deterioration or auto-oxidation), nature and condition of substrates,
required storage time, light, temperature, etc.
Three types of additives
are commonly used to prevent the deterioration of fats and oils: antioxidants,
their synergists, and preservatives (antimicrobial agents).
It should be noted that antioxidants and preservatives act
independently of each other.
Antioxidants are added to fats
and oils in minute quantities, because of cost factors and regulatory
requirements. Another reason is inversion; when an excess of antioxidants acts
in the opposite way and actually promotes oxidation. Antioxidants provide an
alternate path for oxidation which does not involve the substrate, e.g. fats and
oils. The antioxidant does not function indefinitely; it is destroyed in the
process.
Peroxide value is one of the most
important criteria used to monitor the oxidation process. In the course of
oxidation, the peroxide value first increases very slowly (the induction
period). When this period ends, the peroxide value starts growing very rapidly,
and signs of deterioration become evident. The efficacy of antioxidants can be
expressed as a protective factor (PF):
Induction period with
antioxidant
PF = ________________________________
Induction period without antioxidant
This formula shows that the role
of antioxidants is to extend the induction period and thus prolong the product's
shelf life.
The induction period varies for
different fats and oils depending on their chemical composition. Even for the
same fats and oils, it can vary, depending on such pro-oxidative factors as
light, temperature, metal traces, size of contact surface between air and fats,
etc.
Very often, when added in
allowable concentration levels, antioxidants fail to protect fats and oils from
oxidation. Fortunately their action can be improved by the addition of
synergists.
Synergists have little or no
activity alone, but enhance the activity of true antioxidants. The most common
synergists are phosphoric acid, citric acid, ascorbic acid and ascaorbyl
palmitate. The main function of synergists is to form complexes with the
pro-oxidative metal traces that are found in the most fats and oils. Some
synergists also may participate in regeneration of exhausted antioxidants. The
antioxidative action is not reduced until both antioxidant and synergist are
completely consumed.
Obviously, there is no magical
antioxidant formulation to solve the oxidation problem for all fats and oils.
Some of the antioxidants manufactured and used in formulations are:
BHA / BHT / Ethoxyquin /
Propyl Gallate / TBHQ / Tocopherols (See sections
below)
THE USE OF ANTIOXIDANTS AND
ANTIMICROBIALS IN FOOD PRESERVATION
The are two basic ways in which
food products can spoil, or undergo an unwanted chemical change:
1. spontaneous oxidation by atmospheric oxygen
2. chemical reactions catalyzed by microbes
These can include all of biochemistry, hydrolysis, reduction, and not
surprisingly oxidation as well (Hius in't Veld, Leninger).
Food preservation strategies
follows the same logic. Some methods prevent oxidation by adding antioxidants to
be product, others act by eliminating the microbes by heating, irradiation or
antimicrobials. The best methods are those which address both sides of the
problem: hermetic sealing of sterilized food and freezing. The last two
strategies provide a way to prevent chemical changes for a long time, but tend
to be expensive. Since ancient times humanity learned many ways to address the
two sides of food preservation issue inexpensively: if milk is going to sour
anyway - control the process and find uses for sour milk. Fruit will both
oxidize and rot - preserve it by increasing the concentration of acid or sugar
(or both) until neither reaction occur anymore. However in the process, instead
of preserving the original substance, a new food was created. This is not always
an option. A more balanced way of preserving food can be found using modern
knowledge of chemistry.
Antioxidant use alone provides no
antibacterial action. Moreover, antioxidants improve the environment for
bacterial action, because reactive oxygen species are harmful to microorganisms
(Kim et al, Hayashi et al, Stephens et al). Addressing both sides of the food
spoilage problem, addition of a natural antimicrobial substance may not only
help prevent bacterial growth but increase the effectiveness of the antioxidant.
Coca Cola company is adding Phosphoric acid to its trademark beverage for that
reason.
When an abundant source of food
is encountered, bacteria may form communities within a matrix secreted by the
bacteria. More than one species may be included. This biofilm provides a
relatively protected environment for the bacteria (March et al). One of the
goals of food preservation is to prevent the formation of dense bacterial
populations. Isolated pockets of bacterial growth may degrade food even if most
of the food is clean and adequately treated with antioxidants. This is another
reason why initial antimicrobial treatment of food and antioxidation alone may
not be sufficient. A combination of antioxidant and bacteriostatic additives
provides an optimal solution to the problem of preservation of sensitive
substances such as fat and oils.
BHA
BHA can easily by applied to food
items due to its outstanding solubility in fats and oils. BHA adds excellent
stability to food products, fats, oils, vitamins, and pet foods.
|
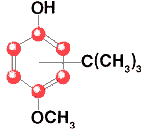
Butylated Hydroxyanisole
C11H16O2
Formula wt 180.25
INS: 320
CAS: [25013-16-5] |
 |
Description
BHA is predominately 3-tert-buryl-4-hydroxyanisole (3-BHA), with varying
amounts of 2-tert-butyl-4-hydroxyanisole (2-BHA). It occurs as a white or
slightly yellow, waxy solid having a faint characteristic odor. It is insoluble
in water, but is freely soluble in alcohol and in propylene glycol. It melts
between 48o and 63o.
Functional Use in
Foods: Antioxidant.
Requirements
Identification: To 5 mL of a 1 in 10,000 solution of the sample in
72% alcohol add 2 mL of sodium borate TS and 1 mL of a 1 in 10,000 solution of
2,6-dichloroquinonechlorimide in absolute alcohol, and mix. A blue color
develops.
Assay: Not less than 98.5% of C11H16O2.
Heavy Metals (as Pb): Not more than 10 mg/kg.
Residue on Ignition: Not more than 0.05%.
Tests
Assay:
Internal Standard Solution: Dissolve about 500 mg of 4-tert-butylphenol,
accurately weighed, in acetone in a 100-mL volumetric flask, add acetone to
volume, and mix.
Standard Preparation: Dissolve together, accurately weighed quantities
of USP reference standards 3-tert-butyl-4-hydroxyanisole and 2-tert-butyl-4-hydroxyanisole
to final concentrations of 9 mg/mL and 1 mg/mL, respectively, in Internal
Standard Solution.
Assay Preparation: Dissolve about 100 mg of Butylated Hydroxyanisole,
accurately weighed, in the Internal Standard Solution in a 10-mL
volumetric flask, dilute with the Internal Standard Solution to volume,
and mix.
Chromatographic System: The gas chromatograph is equipped with a
flame-ionization detector and contains a 1.8-m x 2-mm (id) stainless-steel
column packed with 10% silicone GE XE-60; the column is maintained isothermally
at a temperature between 175o and 185o, and helium is used
as the carrier gas, at a flow rate of 30 mL/min. Chromatograph a sufficient
number of injections of the Standard Preparation, and record the areas as
directed under Procedure, to ensure that the relative standard deviation
does not exceed 2.0% for the 3-tert-butyl-4-hydroxyanisole isomer and
6.0% for the 2-tert-butyl-4-hydroxyanisole isomer; the resolution between
the isomers is not less than 1.3, and the tailing factor doesn't exceed 2.0.
Procedure: Separately inject suitable portions (about 5 mL) of the
Standard Preparation and the Assay Preparation into the gas
chromatograph, and record the chromatograms. Measure the areas under the peaks
for each isomer and the internal standard in each chromatogram, and calculate
the quantity, (I), in mg, of each isomer in the Butylated Hydroxyanisole
by the formula
I = 10 Cs ( Ru
/ Rs )
in which Cs is the
concentration, in mg/mL, of the isomer in the Standard Preparation; Rs
is the ratio of the area of the isomer to that of the internal standard in the
chromatogram from the Standard Preparation; and Ru is the
ratio of the area of the isomer to that of the internal standard in the
chromatogram from the Assay Preparation. Calculate the weight, in mg, C11H16O2
in the Butylated Hydroxyanisole by adding the quantities of the two isomers.
Heavy Metals: Prepare and test a 2-g sample as directed in
Method II under the Heavy Metals Test, using 20 mg of lead ion (Pb) in
the control (Solution A).
Residue on Ignition: Ignite 10 g as directed in the general
method, Appendix IIC.
Packaging and storage
Store in well-closed containers.
BHT
BHT offers excellent solubility
in fats and oils and provides excellent stability in cooked foods. Used alone or
in combination with other antioxidants. It improves stability to fats, oils, and
cereals.
|
Butylated Hydroxytoluene
2,6-Di-tert-butyl-p-cresol
C15H24O
Formula wt 220.35
INS: 321
CAS: [128-37-0] |
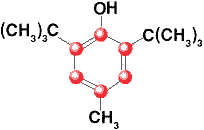 |
Description
A white crystalline solid having a faint characteristic odor. It is insoluble in
water and in propylene glycol, but is freely soluble in alcohol
Functional Use in
Foods: Antioxidant.
Requirements
Identification: To 10 mL of a 1 in 100,000 solution of the sample
in methanol add 10 mL of water, 2mL of sodium nitrite solution (3 in 1000), and
5 mL of dianisidine solution (200 mg of 3,3'-dimethoxybenzidine dihydrochloride
dissolved in a mixture of 40 mL of methanol and 60 mL of 1 N hydrochloric
acid). An orange-red color develops within 3 min. Add 5 mL of chloroform, and
shake. The chloroform layer exhibits a purple or magenta color that fades when
exposed to light.
Assay: Not less than 99.0% of C15H24O.
Heavy Metals (as Pb): Not more than 10 mg/kg.
Residue on Ignition: Not more than 0.002%.
Tests
Assay: Its solidification point is not lower than 69.2o,
indicating a purity of not less than 99.0% of C15H24O.
Heavy Metals: Prepare and test a 2-g sample as directed in
Method II under the Heavy Metals Test, (Appendix IIIB) using 20 �g of
lead ion (Pb) in the control (Solution A).
Residue on Ignition: Transfer a 50-g sample into a tared
crucible, ignite until thoroughly charred, and cool. Moisten the ash with 1 mL
of sulfuric acid, and complete the ignition by heating for 15-min periods at 800o
� 25o to constant weight.
Packaging and storage
Store in well-closed containers.
Ethoxyquin
The best known antioxidant for
animal fats, fish oil and feedstuff. It is available in both liquid and dry
form.
|
Ethoxyquin
6-Ethoxy-1,2-dihydro-2,2,4-trimethylquinoline
C14H19NO
Formula wt, monomer 217.31
INS: 324
CAS: [91-53-2] |
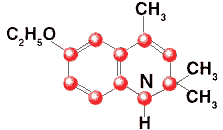 |
Description
Ethoxyquin is a mixture consisting predominantly of the monomer (C14H19NO).
It occurs as a clear liquid that may darken with age without affecting its
antioxidant activity. Its specific gravity is about 1.02, and its refractive
index is about 1.57.
Functional Use in
Foods: Antioxidant.
Requirements
Identification: A solution of 1 mg of the sample in 10 mL of
acetonitrile exhibits a strong fluorescence when viewed under short-wavelength
ultraviolet light.
Assay: Not less than 92.0% of C14H19NO.
Heavy Metals (as Pb): Not more than 10 mg/kg.
Tests
Assay: Transfer about 200 mg of the sample, accurately weighed,
into a 150-mL breaker containing 50 mL of glacial acetic acid, and immediately
titrate with 0.1 N perchloric acid in glacial acetic acid, determining
the endpoint potentiometrically. Perform a blank determination and make any
necessary correction. Each mL of 0.1 N perchloric acid is equivalent to
21.73 mg of C14H19NO(monomer).
Heavy Metals: Prepare and test a 2-g sample as directed in
Method II under the Heavy Metals Test, (Appendix IIIB) using 20 �g of
lead ion (Pb) in the control (Solution A).
Packaging and storage
Store in tightly closed carbon steel or black iron (not rubber, neoprene, or
nylon) containers in a cool, dark place. Prolonged exposure to sunlight causes
polymerization.
Propyl Gallate
The most effective naturally
derived antioxidant for the food industry. Propyl Gallate is made from natural
Gallic Acid, which is obtained by the hydrolysis of tannins from Tara pods. It
exhibits excellent antioxidant activity in food and vegetable oils, especially
in combination with Ascorbyl Palmitate.
|
Propyl Gallate
Gallic Acid, Propyl Ester
C10H12O5
Formula wt 180.20
INS: 310
CAS: [121-79-9] |
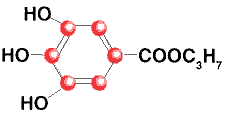 |
Description
A fine, white to nearly white, odorless powder having a slightly bitter taste.
It is slightly soluble in water and freely soluble in alcohol and in either.
Functional Use in
Foods: Antioxidant.
Requirements
Identification: Place about 5 g of the sample and several boiling
chips in a 500-mL round-bottom flask, connect a water-cooled condenser to the
flask, and introduce a steady stream of nitrogen into the flask, maintaining the
flow of nitrogen at all times during the remainder of the procedure. Pour 100 mL
of 1 N sodium hydroxide through the top of the condenser, heat the
solution to boiling, boil for 30 min, and cool. Place the reaction flask in an
ice bath, and slowly, with occasional swirling, add dilute sulfuric acid (10%)
until a pH of 2 to 3 is obtained (using pH paper). Filter the precipitate
through a sintered-glass crucible, wash with a minimum amount of water, and dry
at 110o for 2 h. The gallic acid so obtained melts at about 240o
with decomposition.
Assay: Not less than 98.0% and not more than 102.0% of C10H12O5
after drying.
Heavy Metals (as Pb): Not more than 10 mg/kg.
Loss on Drying: Not more than 0.5%.
Melting Range: Between 146o and 150o.
Residue on Ignition: Not more than 0.1%.
Tests
Assay: Transfer about 200 mg, previously dried at 110o
for 4 h and accurately weighed, to a 400-mL beaker, dissolve it in 150 mL of
water, and heat to boiling. With constant and vigorous stirring, add 50 mL of
bismuth nitrate TS, continue stirring and heating until precipitation is
complete, and cool. Filter the yellow precipitate on a tared sintered-glass
crucible, wash it with cold dilute nitric acid (1 in 300), and dry at 110o
to constant weight. The weight of the precipitate so obtained, multiplied by
0.4866, represents its equivalent of C10H12O5.
Heavy Metals: Prepare and test a 2-g sample as directed in
Method II under the Heavy Metals Test, (Appendix IIIB) using 20 �g of
lead ion (Pb) in the control (Solution A).
Loss on Drying: Appendix IIC - Dry at 110o for 4 h.
Melting Range: Determine as directed for Melting Range or
Temperature, Appendix IIB, after drying at 110o for 4 h.
Residue on Ignition: Appendix IIC - Ignite 2 g as directed in
the general method.
Packaging and storage
Store in well-closed containers.
TBHQ
TBHQ is an antioxidant which
offers excellent solubility in fats and oils. Used alone or in combination with
other antioxidants it adds stability to fats, oils, cereals and packaging
materials.
|
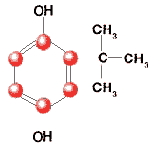
TBHQ
tert-Butylhydroquinone; Mono-tert-butylhydroquinone
C10H14O2
Formula wt 166.22
INS: 319
CAS: [1948-33-0] |
 |
Description
A white, crystalline solid having a characteristic odor. It is soluble in
alcohol and in ether, but is practically insoluble in water.
Functional Use in Foods: Antioxidant.
Requirements
Identification: Dissolve a few mg of the sample in 1 mL of
methanol, and add a few drops of a 25% solution of dimethylamine in water. A
pink to red color is produced.
Assay: Not less than 99.0% of C10H14O2.
t-Butyl-p-benzoquinone: Not more than 0.2%
2,5-Di-t-butylhydroquinone: Not more than 0.2%
Heavy Metals (as Pb): Not more than 10 mg/kg.
Hydroquinone: Not more than 0.1%.
Melting Range: Between 126.5o and 128.5o.
Toluene: Not more than 0.0025%.
Ultraviolet Absorbance (polynuclear hydrocarbons): Passes test.
Tests
Assay: Transfer about 170 mg of the sample. previously ground
to a fine powder and accurately weighed, into a 250-mL, wide-mouth Erlenmeyer
flask, and dissolve in 10 mL of methanol. Add 150 mL of water, 1mL of 1 N
sulfuric acid, and 4 drops of diphenylamine indicator (3 mg of p-diphenylaminesulfonic
acid sodium salt per mL of 0.1 N sulfuric acid), and titrate with 0.1
N ceric sulfate to the first complete color change from yellow to red
violet. Record the volume, in mL, of 0.1 N ceric sulfate required as V.
Calculate the percentage of C10H14O2 in the
sample, uncorrected for hydroquinone (HQ) and 2,5-di-tert-butylhydroquinone
(DTBHQ), by the formula
8.311N ( V - 0.1 mL
) / W
in which 0.1 mL represents the
volume of ceric sulfate solution consumed by the primary oxidation products of
tert-Butylhydroquinone ordinarily present in the sample; N is the exact
normality of the ceric sulfate solution; and W is the weight of the sample
taken, in g. Record the uncorrected percentage thus calculated as A. If
HQ and DTBHQ are present in the sample, they will be included in the titration.
Calculate the corrected percentage of C10H14O2
in the sample by the formula
A
- ( % HQ x 1.51 ) - ( % DTBHQ x 0.75)
using the respective values for
percentage of HQ and percentage of DTBHQ as determined under 2,5-Di-t-butylhydroquinone
and Hydroquinone.
t-Butyl-p-benzoquinone:
Standard Preparation Transfer about 10 mg of FCC
Mono-tertiary-butyl-p-benzoquinone Reference Standard, accurately
weighed, into a 10-mL volumetric flask, dissolve in chloroform, dilute to volume
with the same solvent, and mix.
Sample Preparation Transfer about 1 g of the sample, previously
reduced to a fine powder in a high-speed blender and accurately weighed, into a
10-mL volumetric flask, dilute to volume with chloroform, and shake for 5 min to
extract the t-butyl-p-benzoquinone. Filter through a Millipore
filter or equivalent, before use in the Procedure below.
Procedure Fill the reference cell with chloroform and the sample cell
with the Standard Preparation, place the cell in the respective reference and
sample beams of the spectrophotometer, and record the infrared spectrum from
1600 to 1775 cm-1. On the Spectrum draw a background line from 1612
to 1750 cm-1, and determine the net absorbance (AS) of the
standard Preparation at 1659cm-1. Similarly, obtain the spectrum of
the Sample Preparation, and determine its net absorbance (AU) at 1659 cm-1.
Calculate the percentage of t-butyl-p-benzoquinone in the sample
by the formula
100 x ( AU / AS
) x ( WS / WU )
in which WS is the
exact weight, in mg, of the Reference Standard taken, and WU is the
exact weight, in mg, of the sample taken.
2,5-Di-t-butylhydroquinone and Hydroquinone:
Stock Solutions Weigh accurately about 50 mg each of hydroquinone
(HQ), 2,5-di-t-butylhydroquinone (DTBHQ), and methyl benzoate (internal
standard), transfer into separate 50-mL volumetric flasks, dilute to volume with
pyridine, and mix.
Calibration Standards Into separate 10-mL volumetric flasks add 0.50,
1.00, 2.00, and 3.00 mL of the HQ stock solution, then to each flask add 2.00 mL
of the methyl benzoate (internal standard) stock solution, dilute each to volume
with pyridine, and mix. In the same manner prepare four DTBHQ calibrating
solutions. Prepare the trimethylsilyl derivative of each solution as follows:
Add 9 drops of calibration solution to a 2-mL serum vial, cap the vial, evacuate
with a 50-ml gas syringe, add 250 �L of N,O-bis-trimethylsilylacetamide,
and heat at about 80o for 10 min. Chromatograph 10-�L portions of
each standard in duplicate, and plot the concentration ratio of HQ to internal
standard (X-axis) against the response ratio of HQ to internal standard
(Y-axis). Plot the same relationships between DTBHQ and the internal standard.
Sample Preparation and Procedure Transfer about 1 g of the sample,
accurately weighed, into a 10-mL volumetric flask, add 2.00 mL of the methyl
benzoate internal standard stock solution, dilute to volume with pyridine, and
mix. Prepare the trimethylsilyl derivative as described above under
Calibration Standards, and then chromatograph duplicate 10-�L portions to
obtain the chromatogram. The approximate peak times, in min, are methyl
benzoate, 2.5; TMS derivative of HQ, 5.5; TMS derivative of tert-Butylhydroquinone,
7.3; TMS derivative of DTBHQ, 8.4.
Calculation Determine the peak areas (response) of interest by automatic
integration or manual triangulation. Calculate the response ratio of HQ and
DTBHQ to internal standard. From the calibration curves determine the
concentration ratio of HQ and DTBHQ to internal standard, and calculate the
percentage of HQ and the percentage of DTBHQ in the sample by the formula
A = Y x I x 10 / S
in which A is the
percentage of HQ or the percentage of DTBHQ in the sample; Y is the
concentration ratio (X-axis on calibration curve); I is the percentage
(w/v) of internal standard in the Sample Preparation; and S is the weight
of sample taken, in g.
Packaging and storage Store in well-closed containers.
Reference: FCC IV
Tocopherols
The most commonly used natural
antioxidant for foodstuffs, pharmaceuticals and cosmetics. Natural tocopherols
are obtained as an ancillary product in the production of vegetable oils. They
are relatively mild compounds, suitable for use in food products, cosmetics, and
those vegetable oils which have low tocopherol content.
|
Tocopherol
C29H50O2
Formula wt 430.71
INS: 307
CAS: [2074-53-5] |
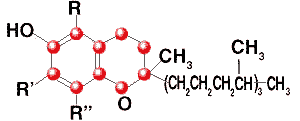
Natural Mixture of alpha,
beta, gamma and delta tocopherols |
Description
A form of vitamin E. It occurs as a yellow to amber, nearly odorless, clear,
viscous oil that oxidizes and darkens in air and on exposure to light. It is
insoluble in water, is freely soluble in alcohol, and is miscible with acetone,
chloroform, ether, fats, and vegetable oils.
Functional Use in
Foods: Nutrient; dietary supplement; antioxidant.
Requirements
Labeling: Label claims in terms of former International Units (IU)
should be based on the following 1 mg DL-a-tocopherol = 1.1 IU
Identification:
A. Dissolve about 10 mg of the sample in 10 mL of absolute alcohol, add
with swirling 2 mL of nitric acid, and heat at about 75o for 15 min.
A bright red to orange color develops.
B. The retention time of the major peak (excluding the solvent peak) in
the chromatogram of the Assay Preparation is the same as that of the
Standard Preparation, both relative to the internal standard, as obtained in
the Assay.
C. If the isomeric form is not otherwise known, determine the optical
rotation on a 1 in 10 solution of the sample in chloroform. The specific
rotation is not appreciable (approximately +/- 0.05o).
Assay: Not less than 96.0% and not more than 102.0% of C29H50O2.
Heavy Metals (as Pb): Not more than 10 mg/kg.
Acidity: Passes test.
Tests
Note: In the following Assay, use low-actinic glassware for all
solutions containing tocopherols.
Assay:
Internal Standard Solution: Prepare a solution in n-hexane
containing 3 mg of hexadecyl hexadecanoate, accurately weighed, in each mL
Standard Preparation: Dissolve about 30 mg of USP Alpha Tocopherol
Reference Standard, accurately weighed, in 10.0 mL of the Internal Standard
Solution.
Assay Preparation: Dissolve about 30 mg of the sample,
accurately weighed, in 10.0 mL of the Internal Standard Solution.
Chromatographic System: Use a gas chromatograph equipped with a
flame-ionization detector and a glass-lined sample-introduction system or
on-column injection. Under typical conditions, the instrument contains a 2-m x
4-mm borosilicate glass column packed with 2% to 5% methylpolysiloxane gum on
80- to 100-mesh acid-base washed silanized chromatographic diatomaceous earth.
The column is maintained isothermally between 240o and 260o,
the injection port at about 290o, and the detector block at about 300o.
The flow rate of dry carrier gas is adjusted to obtain a hexadecyl hexadecanoate
peak approximately 10 to 20 min after sample introduction when a 2% column is
used, or 30 to 32 min when a 5% column is used.
Note: Cure and condition the column as necessary.
System Suitability: Chromatograph a sufficient number of injections of
a mixture in n-hexane of 1 mg/mL each of USP Alpha Tocopherol Reference Standard
and USP Alpha Tocopheryl Acetate Reference Standard, as directed under
Calibration, to ensure that the resolution factor, R, is not less
than 1.0.
Calibration: Chromatograph successive 2- to 5-mL portions of
the Standard Preparation until the relative response factor, F. is
constant (i.e., within a range of approximately 2%) for three consecutive
injections. If graphic integration is used, adjust the instrument to obtain at
least 70% maximum recorder response for the hexadecyl hexadecanoate peak.
Measure the areas under the major peaks occurring at relative retention times of
approximately 0.51 (a-tocopherol) and 1.00 (hexadecyl hexadecanoate), and record
the values as AS and AI, respectively. Calculate the
relative response factor, F, by the formula
( AS / AI
) x ( CI / CS )
in which CI and CS
are the exact concentrations, in mg/mL, of hexadecyl hexadecanoate and of USP
Alpha Tocopherol Reference Standard in the Standard Preparation,
respectively.
Procedure: Inject a suitable portion (2 to 5 mL) of the Assay
Preparation into the chromatograph, and record the chromatogram. Measure the
areas under the major peaks occurring at relative retention times of
approximately 0.51 (a-tocopherol) and 1.00 (hexadecyl hexadecanoate), and record
the values as aU and aI, respectively. Calculate the
weight, in mg, of DL-a-tocopherol in the sample by the formula
( 10CI / F ) x ( aU
/ aI )
Heavy Metals:
Place a 2-g sample in a silica crucible, and proceed as directed in Method II
under the Heavy Metals Test, Appendix IIIB, using 20 mg of lead ion (Pb)
in the control (Solution A).
Acidity: Dissolve 1.0 g of the sample in 25 mL of a mixture of
equal volumes of alcohol and ether that has been neutralized to phenolphthalein
TS with 0.1 N sodium hydroxide, add 0.5 mL of phenolphthalein TS, and
titrate with 0.1 N sodium hydroxide until the solution remains faintly
pink after shaking for 30 s. Not more that 10 mL of 0.1 N sodium
hydroxide is required.
-
2-Methoxyethanol (EGME)
-
Ethylene Glycol Monomethyl Ether (EGME)
| 

 Plastics Engineering
Plastics Engineering


 Knowledge Base
Knowledge Base

















 Knowledge Base
Knowledge Base


